A boy with severe epilepsy has become the first patient in the world to trial a new device fitted in his skull to control seizures. The neurostimulator, which sends electrical signals deep into his brain, has significantly reduced Oran Knowlson’s daytime seizures by 80%.
Oran’s mother, Justine, shared with the BBC that her son is now happier and enjoying a much better quality of life. The surgery took place in October 2023 at Great Ormond Street Hospital in London when Oran, now 13, was 12.
Oran, from Somerset, suffers from Lennox-Gastaut syndrome, a treatment-resistant form of epilepsy that began at age three. Prior to the surgery, Oran experienced several daily seizures, sometimes hundreds, severely affecting his life. Justine described the condition as having robbed Oran of his childhood, causing a rapid deterioration and loss of skills.
The CADET Project
Oran is part of the CADET project, a series of trials assessing the safety and effectiveness of deep brain stimulation for severe epilepsy. This initiative involves Great Ormond Street Hospital, University College London, King’s College Hospital, and the University of Oxford. The Picostim neurotransmitter used in the trial is made by the UK company Amber Therapeutics.
How the Device Works
Epilepsy seizures are triggered by abnormal bursts of electrical activity in the brain. The Picostim device emits a constant pulse of current, aiming to block or disrupt these abnormal signals. Consultant paediatric neurosurgeon Martin Tisdall led the team that inserted two electrodes deep into Oran’s brain, reaching the thalamus, a key relay station for neuronal information. The neurostimulator, a 3.5cm square device, was placed in a gap in Oran’s skull and anchored in place.
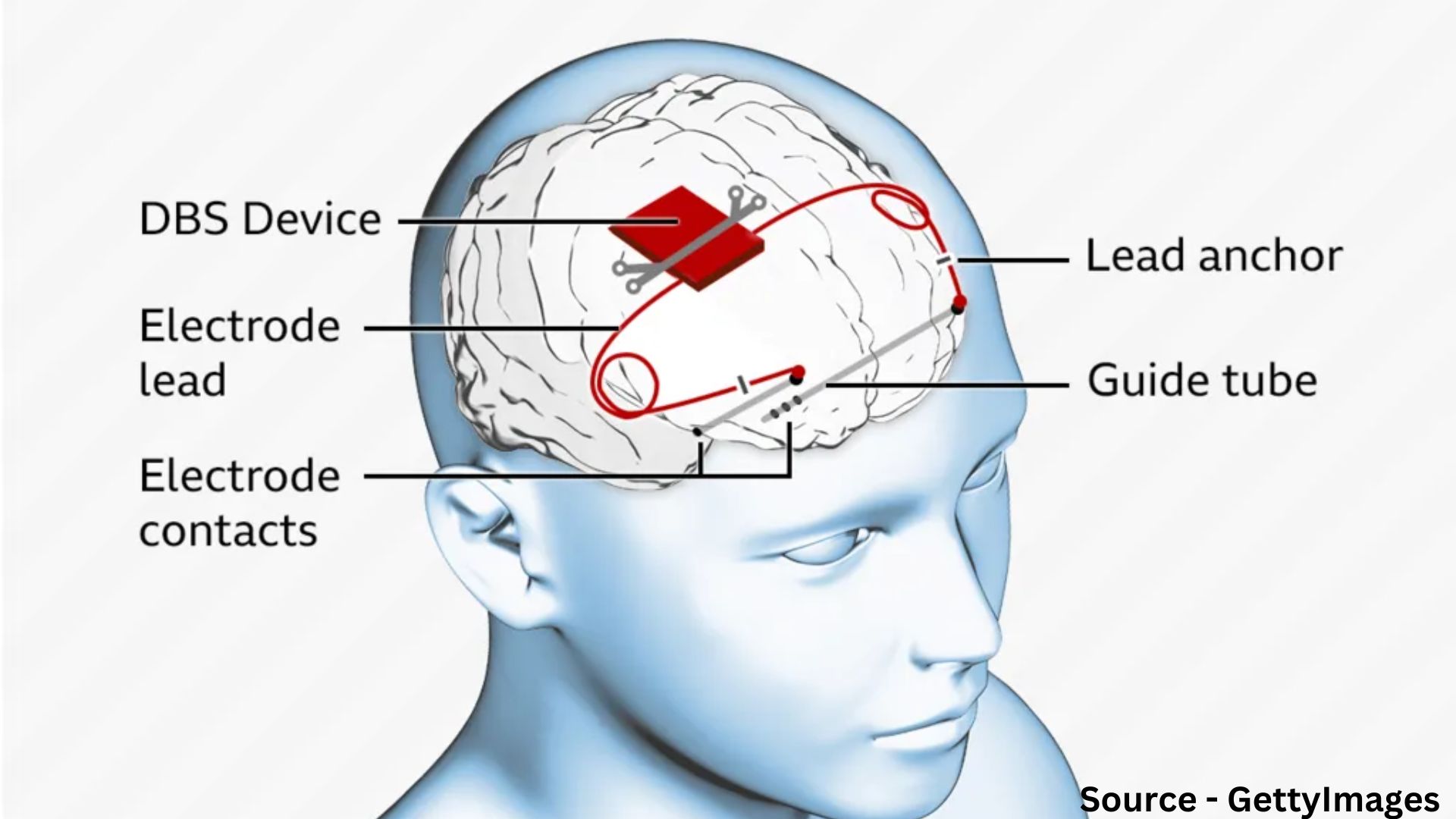
Deep brain stimulation has been attempted before for childhood epilepsy, but previous neurostimulators were placed in the chest with wires running to the brain. This new approach, with the device implanted in the skull, reduces potential complications such as infections and device failures.
Oran had a month to recover before the neurostimulator was activated. He cannot feel the device when it is on and can recharge it daily via wireless headphones. Seven months post-operation, Justine reported a massive improvement in Oran’s condition: he is more alert with no daytime drop seizures, and his nighttime seizures are shorter and less severe.
Currently, Oran receives a constant electrical stimulus from the device. The next phase of the trial aims to make the neurostimulator respond in real time to changes in brain activity, potentially blocking seizures as they occur. Justine expressed her excitement about this development, noting that the Great Ormond Street team has given them hope for a brighter future.
As part of the trial, three more children with Lennox-Gastaut syndrome will be fitted with the deep brain neurostimulator. While Oran’s treatment is not a cure, his family is optimistic about continued improvements in his condition.
The Picostim neurostimulator, owned by Amber Therapeutics, has also been used to treat patients with Parkinson’s disease. In the United States, another type of skull-mounted neurostimulator has been used to treat epilepsy, indicating a growing interest in this innovative approach to managing neurological disorders.
-Source BBC